Cancel 2018. 3
<-- sched 2, MJ sched 1
great, dunceler's inner retard is showing again...let us all thank onceler for bringing up race in every thread on the board
Huh? WTF are you talking about. Hell all I asked for was peer reviewed data.lol....tom completely pwned legion and mopp
notice how mott was quick to accept the OP, but when tom posted another study, he all of a sudden wants "peer" review....
ROTFLMOA!!!!!!!!!!!!!!Shouldn't you be starting a thread about what would happen if the peer review was by a black guy about white guys?
Just curious... is the above peer reviewed by peers that have been properly vetted and reviewed? I demand that the peers able to peer review something be reviewed to make sure their reviews are going to fit the fear mongering I wish to do.
well this post should bring out the science deniers again.
Huh? WTF are you talking about. Hell all I asked for was peer reviewed data.
Yes, I am perfectly aware of the effect of pH on biogenic calcification especially corals.
The amount of CO[SUB]2[/SUB] that is dissolved in water depends on the temperature of the water, and on the concentration present in the water and the pressure of CO[SUB]2[/SUB] in the air above the water. The warmer the water, the less CO[SUB]2[/SUB] will dissolve in it. Because cold water will absorb more carbon dioxide, it follows that if water containing dissolved CO[SUB]2[/SUB] is warmed it will release CO[SUB]2[/SUB] into the air. (Consider the effect when a warm bottle of beer is opened!) The more CO[SUB]2[/SUB] that is present in the air above the water, the more CO[SUB]2[/SUB] that will dissolve.
I wouldn't go down the "conspiracy theory" road if I was you Tom. Peer review, as I'm sure you know, means that your work is submitted to qualified academic peers in that area of research to review. If the findings are accepted then the research is published. It is hardly a "good ole boy" process as those peers have no vested interest in your research. In fact, it's usually the opposite as peer review is most often quite competitive with comptetive researchers publishing competitive findings and conclusion. That makes building a consensus in the scientific community of anything exceedingly difficult.I do often wonder how the peers who do the reviewing are chosen, it does seem to be a bit of an old boy's network at times.
Do you deny that there are a lot of people who deny the scientific consensus about anthroprogenic climate change?nice try. that is your first post....
I wouldn't go down the "conspiracy theory" road if I was you Tom. Peer review, as I'm sure you know, means that your work is submitted to qualified academic peers in that area of research to review. If the findings are accepted then the research is published. It is hardly a "good ole boy" process as those peers have no vested interest in your research. In fact, it's usually the opposite as peer review is most often quite competitive with comptetive researchers publishing competitive findings and conclusion. That makes building a consensus in the scientific community of anything exceedingly difficult.
That also means that the scientific conspiracy theorist are mostly just plain wacko.
Do you deny that there are a lot of people who deny the scientific consensus about anthroprogenic climate change?
Conversely, for you science deniers, why interrupt good old fashioned political hackery with objective science.
Show me the peer reviewed data. If it does provide compelling evidence of macroscopic changes in oceanic pH then that would be legitimage cause for concern. My concern here would not be the climactic implications but rather the biological ones.
I wouldn't go down the "conspiracy theory" road if I was you Tom. Peer review, as I'm sure you know, means that your work is submitted to qualified academic peers in that area of research to review. If the findings are accepted then the research is published. It is hardly a "good ole boy" process as those peers have no vested interest in your research. In fact, it's usually the opposite as peer review is most often quite competitive with comptetive researchers publishing competitive findings and conclusion. That makes building a consensus in the scientific community of anything exceedingly difficult.
That also means that the scientific conspiracy theorist are mostly just plain wacko.
Anthropogenic climate change Tinkerbell.Tell us Mutt... what 'Science" am I denying?
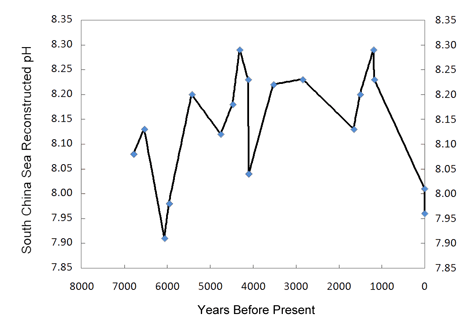
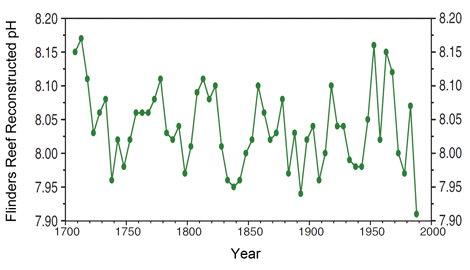
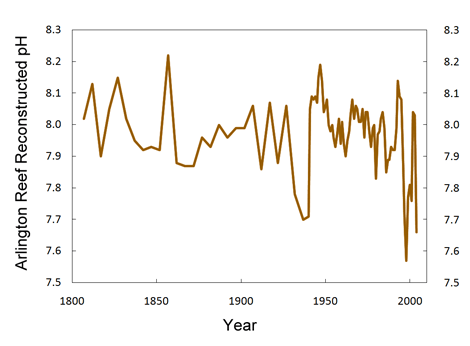
I think you're misinterpreting this article. It doesn't dispute oceanic acidification at all. This article is proposing that with out an understanding of environmental variability of oceanic pH that there is a gap in the current data on the biological and ecological consequences of oceanic acidification on biogenic calcifiation. In fact the bent of this article is the development of a method that can measure this environmental variability. Again, this article does not dispute oceanic acidification.
Yes, yes this is just some grand conspiracy by thousands of competing scientist from all over the world who are focused on denying you your right to drive your F-150. I do see your point.LOL you worship scientists. they're just people, dude. they lie cheat and steal just like everyone
I think you're misinterpreting this article. It doesn't dispute oceanic acidification at all. This article is proposing that with out an understanding of environmental variability of oceanic pH that there is a gap in the current data on the biological and ecological consequences of oceanic acidification on biogenic calcifiation. In fact the bent of this article is the development of a method that can measure this environmental variability. Again, this article does not dispute oceanic acidification.
Well yes certainly more research needs to be done. No one is disputing that and I do understand your question. However your talking about a solution in equilibrium. The ocean is hardly that.Yes, it is saying that the current knowledge is inadequate and needs far more research. One thing I have never been able to understand though is how can more CO2 be absorbed by the oceans if they get warmer due to global warming? The solubility of gases are inversely proportional to the temperature so there ought to be less not more absorption.
Yes, it is saying that the current knowledge is inadequate and needs far more research. One thing I have never been able to understand though is how can more CO2 be absorbed by the oceans if they get warmer due to global warming? The solubility of gases are inversely proportional to the temperature so there ought to be less not more absorption.
Does this take into account absorbtion by flora and fauna?